Introduction
The sessile nature of plants has provided for unique adaptations to their surrounding environments. Over their evolutionary course, plants have survived by changing their functional or structural properties. Animals, being mobile, can change their circumstances to some extent, but plants can not. Plants are not, however, static; they are able to respond to a variety of environmental stimuli. Plants perform various macroscopic and microscopic movements in response to intrinsic and extrinsic stimuli.
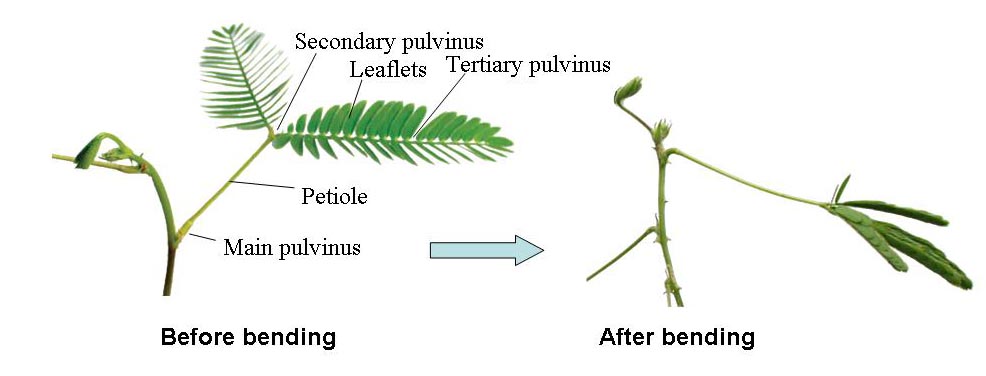
Mimosa pudica L. responds to mechanical, thermal, electrical, and chemical stimuli with rapid bending of leaves and petioles. Like those of other Mimosa family members, leaves of M. pudica close at night or in darkness and open during the day or in light. Thus, the sensitive M. pudica has the ability to perform both seismonastic and nyctinastic movements. Movement of leaves and leaflets in Mimosa is driven by changes in the curvature of the pulvinus. Folding of leaves and bending of petioles are caused by rapid shrinkage of the lower side of motor cells of the pulvinus. Stimulation of a leaf causes an electrical signal to spread up the petiole. Subsequently, the electrical signal turns into a chemical signal that makes certain cell membranes become more permeable to K+ and Cl- ions. The increased permeability of the cell membranes causes ions to move out from the symplast into the apoplast. Water accompanies the ions into the apoplast by osmosis. This phenomenon is known as plasmolysis. As a result of plasmolysis, the protoplast will shrink, which makes the leaflets or petioles droop.
REGULATORY MECHANISM
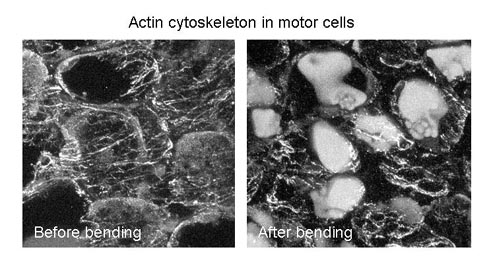
We have investigated the role of the cell cytoskeleton during seismonastic movement of Mimosa. Plant cytoskeletons participate in fundamental processes including mitosis, cytokinesis, cell polarity and intracellular trafficking. By treating the motor organ with reagents that interfere with actin, such as cytochalasin B and phalloidin, Fleurat-Lessard and colleagues found that the loss of ability of the main pulvinus to bend, suggesting that rearrangement of the actin cytoskeleton is involved in seismonastic movement. We also have shown that fragmentation of actin filaments and reductions in the levels of the most acidic forms of actin are associated with bending of the petiole and that this phenomenon is prevented by Tyr phosphatase inhibitors. Thus, we believe that a dynamic reorganization of the actin cables that form the cell cytoskeleton occurs during seismonastic movement and that inhibition of Tyr dephosphorylation of actin prevents reorganization of the actin cytoskeleton, suggesting that the Tyr phosphorylation/dephosphorylation cycle of actin plays a pivotal role in the seismonastic movement of Mimosa.
ACTIN BINDING PROTEINS
Recent investigations have focused on molecular mechanisms regulating the actin cytoskeleton in the motor cells. In animals, formation and stability of actin filaments is controlled by actin-binding proteins that affect actin nucleation, actin monomer sequestration, and actin filament (F-actin) severing, capping, or bundling. Several actin-binding proteins have also been identified biochemically and molecular biologically in plants. They are thought to be responsible for modulating changes in actin organization and the dynamics of plant cell morphogenesis and development. We have shown that the presence of a protein similar to the gelsolin family proteins that severs actin filaments in a Ca2+-dependent manner. We also isolated annexin from the plant. Plant annexins are distributed in a wide variety of tissues and are able to bind actin in a calcium- or pH-dependent manner. Mimosa annexin is localized to the plasma membrane of pulvinar cells during the daytime. However, we observed a drastic change in distribution at night. Thus, Mimosa annexin appears to contribute to membrane reorganization during nyctinastic movements. In higher plants, villin, which was originally isolated from the actin bundles of intestinal epithelial cell microvilli, promotes formation of stable actin cables in vivo and in vitro (Yokota & Shimmen). Our present observations suggest that an actin-bundling protein is involved in the formation of actin cables, and the activity of such a protein appears to be regulated in response to external stimuli. So far, we focus on the contribution of villin in the seismonastic movement.
AQUAPORINS
As I mentioned above, an increased permeability causes water and ions to move from the symplasts into the apoplasts. Over the course of a few seconds, water in the extensor half of the pulvinus is transferred to the flexor half, resulting in movement of the leaf. Thus, water tansport is a key factor for the initiation of the seismonastic movement. Aquaporins (AQPs) are water channels that influence changes in turgor pressure in plant cells by transporting water across cell membranes. We isolated cDNAs encoding Mimosa aquaporins and expressed these clones in Xenopus oocytes. Mimosa aquaporins PIP2;1 and TIP1;1 show high water channel activity. When both clones were coexpressed in Xenopus oocytes, the water channel activity of the oocytes were increased rather than that of single expression. We also found that only PIP1;1 was phosphorylated by PKA at Serine131, and this phosphorylation enhanced the cooperative regulation of PIP isoforms. Thus, we believe that the permeability of membranes of Mimosa to water is regulated by both direct interactions and phosphorylation of AQP isoforms.
