Department of Materials and Life Sciences, Faculty of Science and Technology, Sophia University
有機合成化学グループ: 鈴木(教)研究室(&旧 増山研究室)
abstracts_2016_2
| Catalytic Availabilities of Lewis Acidic SnCl2: Ph3PAuCl-SnCl2 Composite-Catalyzed Successive ortho-Alkenylation/O-Alkenylation of Phenols Followed by Cyclization to 1-Benzopyrans Atsuchi Tochigi, Kadzuoki Tsukamoto, Noriyuki Suzuki,* Yoshiro Masuyama* Eur. J. Org. Chem. 2016, 5678-5685. DOI: 10.1002/ejoc.201601042 |
Ph3PAuCl-SnCl2composite, prepared beforehand insitu from Ph3PAuCl and SnCl2, catalyzed the preparationof 1-benzopyrans from phenols and phenylacetylene. The Ph3PAuCl-SnCl2composite-catalyzed reaction was presumed to proceed via the successiveortho-alkenylation andO-alkenylation of phenols withphenylacetylene followed by the cyclization of preparedortho- andO-dialkenylatedphenol derivatives. Conveniently, all reactions with Ph3PAuCl-SnCl2composite were established without much attention to air and water. Ph3PAuCl-catalyzed nucleophilic additions of phenols tophenylacetylene with SnCl2as a Lewis acidic auxiliary agent; Ph3PAuCl-SnCl2composite-catalyzed successiveortho-alkenylation[9,10]andO-alkenylation[11,12]of phenols followed by cyclization to 1-benzopyrans.[13, 14]In addition, theortho-alkenylationcatalyzed by SnCl2itself is described, which has been found in thecourse of the study of Ph3PAuCl-SnCl2compositecatalysis. |
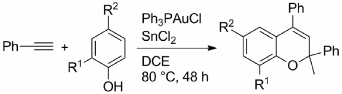 |
| ルイス酸性塩化スズSnCl2(II)ノ触媒活性: Ph3PAuCl-SnCl2 が触媒する連続したフェノールのオルト-アルケニル化/O-アルケニル化、それに続く環化による1-ベンゾピラン類の合成 栃木 淳志, 塚本 一興, 鈴木 教之,* 増山 芳郎* Eur. J. Org. Chem. 2016, 5678-5685. DOI: 10.1002/ejoc.201601042 |
| 金錯体と塩化スズ(II)から構成される系が触媒として機能し、フェノールとフェニルアセチレンから1-ベンゾピラン類を与えることを見出した。反応は、フェノールのオルト―アルケニル化と続くO-アルケニル化、それに続く環かによって進行した。 |
