Department of Materials and Life Sciences, Faculty of Science and Technology, Sophia University
有機合成化学グループ: 鈴木(教)研究室(&旧 増山研究室)
abstracts_2023_2
| Nucleophilic Carbon-Carbon Bond Formation by 1-En-3-ynes via Zircocnocene-η2-1-en-3-yne Complexes. Noriyuki Suzuki*, Saki Ito, Mari Kawate, Takahiko Takemoto Journal of Organometallic Chemistry, 2022, 982, 122531. DOI: 10.1016/j.jorganchem.2023.122883 |
| Abstract: Nucleophilic reactions by the C1 atom of 1-en-3-ynes toward organic electrophiles such as ketones, nitriles and isocyanates, via their organozirconium species, were investigated. Zirconocene complexes of conjugated 1-en-3-ynes in a η2-fashion were prepared, and they reacted with ketones, nitriles and isocyanates to afford seven-membered intermediate organozirconium compounds via C-C bond formation, and the corresponding alcohols, ketones, pyrroles and amides after hydrolysis. The products were, in principle, the same as those obtained from five-membered zirconacycloallene complexes, which were prepared from low-valent zirconocene and 1-en-3-ynes in the absence of ancillary ligands. Because only a limited number of 1-en-3-ynes can form zirconacycloallenes in significant yields, the present study allowed a wider scope of 1-en-3-ynes in this synthetically useful reaction. |
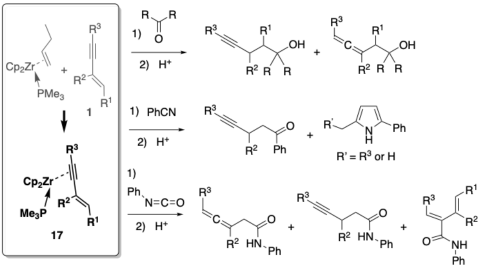 |
| ジルコノセン-1-エン-3-イン錯体を経由する、共役エンインによる求核的な炭素-炭素結合生成反応 鈴木 教之,* 伊東沙姫, 川手真理, 竹元貴彦 Journal of Organometallic Chemistry, 2023, in press. DOI: 10.1016/j.jorganchem.2023.122883 |
| 共役エンインのη2-ジルコノセン錯体を合成し、ケトン、ニトリル、イソシアナートなどの求電子剤との反応を検討した。五員環アレンを形成しにくい共役エンインにおいても、錯体17を経由することにより同様の反応が進行することが確認できた。 |
