Department of Materials and Life Sciences, Faculty of Science and Technology, Sophia University
有機合成化学グループ: 鈴木(教)研究室(&旧 増山研究室)
abstracts_2021_2
| Reactions of Five-Membered Zirconacycloalkynes And Zirconacycloallenes with Cp2Zr(H)Cl; Formal Hydrogenation by Metal Hydrides. Noriyuki Suzuki,* Sayaka Ban, Ayari Mochizuki, Saki Ito Dalton Transactions, 2021, 50, 16262-16272. DOI: 10.1039/D1DT03313A |
| Reactions of five-membered zirconacycloalkynes and zirconacyloallenes (1-zirconacyclopent-3-ynes and 1-zirconacyclopenta-2,3-dienes) with an excess of Cp2Zr(H)Cl, known as the Schwartz reagent, was studied. Both reactions gave five-membered zirconacycloalkenes, 1-zirconacyclopent-3-enes without subsequent work-up such as protonolysis and hydrogenolysis. The product was identical to the zirconocene-diene complex that was prepared from Cp2Zr(n-Bu)2 (Negishi reagent) and the corresponding 1,4-disubstituted 1,3-dienes. These results indicate that formal hydrogenation by metal hydride took place. The use of diisobutylaluminum hydride or 9-borabicyclo[3.3.1]nonane also gave the same product, albeit in lower yields. The reactions starting from deuterated compounds suggested that double hydrozirconation followed by elimination of a dinuclear zirconium complex resulted in the hydrogenated products. |
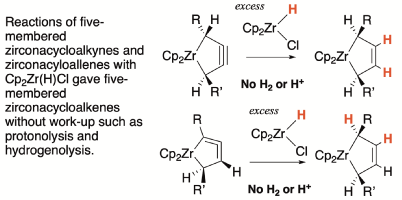 |
| 有機ジルコニウム五員環アルキン、五員環アレンとCp2Zr(H)Clの反応:金属ヒドリドによる水素化反応 鈴木 教之,* 伴 沙弥果, 望月 菖里, 伊東 沙姫 Dalton Transactions, 2021, 50, 16262-16272. DOI: 10.1039/D1DT03313A |
| 有機ジルコニウム化合物である五員環アルキン、五員環アレン錯体とCp2Zr(H)Clの反応を検討した。反応はいずれも五員環アルケン錯体を与え、ジルコノセン-ジエン錯体に相当する化合物であった。重水素化錯体を用いた実験から、反応は2回のヒドロジルコネーションの後2核Zr錯体が脱離する機構が支持された。 |
